Proteins
We work on proteins (and their environment)
Proteins are the core of life, they do all the work, and they give you
feelings, contact with the outside world, etc. Proteins, therefore, are
the most important molecules on earth.
We want to understand life; why are we what we are, why do we do what
we do, how come you can think what you think? Therefore we must understand
proteins and all our work is aimed
at just that: understanding proteins. We want to use this understanding to
improve the world around us in a careful and responsible manner.
Can we make medicines that can cure diseases by making small molecules
that interact with proteins? And if we have found such a medicine, can we
make a similar one that works better, faster, or with less side effects?
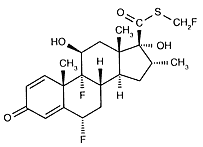
|
I use fluticason every day to be able to breath at night.
Since I started using this medicine, I sleep much better. This is
important, but this medicine has some side effects. Not much, not
scary ones. But it would still be good to find a better molecule that
makes me breath well, without those possible side effects.
|
|
Only few of us like it to visit the dentist. But there is hope.
One of these days, engineered bacteria are no longer considered scarry,
and we can start using engineered bacteria that prevent caries.
Obviously, we need to know which proteins are the
dangerous ones, what is their role in the whole bacterium, and we need to
know that protein's structure before we can start engineering it.
|
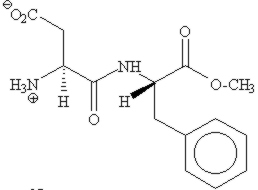
|
Overweight is a big problem in the modern society.
Nutrasweet is a low calory, atrificial sweetener that helps people keep their
weight down by reducing their calory intake.
|
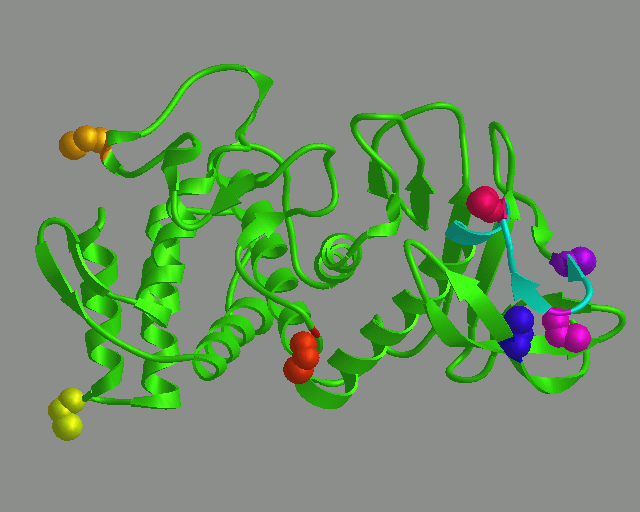
|
Nutrasweet is produced by neutral proteases. After many years of modelling,
mutation studies, and protein chemistry experiments, we designed a neutral
protease that can produce neutrasweet faster, and probably also cleaner.
|
Unfortunately, there is a irrational large scale anti genetic-engineering
attitude among the population, so our protease that would make diet sodas
cheaper and produced with much less pollution will not be used. Still,
bioinformatics and protein engineering can do such things when the protein
structure is known, or can be modelled.
Lets hope these few examples are enough to convince you that proteins are
worth studying. You can also look at a few more examples that we actually
work(ed) on.
This introduction, of course, is biased. And many people will disagree.
But in our group, this is why we do what we do.
Most time in the group is spent on developing new techniques, but there is
no fun in that if those techniques aren't used to do what we really like
to do: Understanding life, or more down to earth, solving problems brought
to us by our friends in biology, biochemistry, cell biology, medicine,
antropogenetics, molecular biology, immunochemistry, etc.
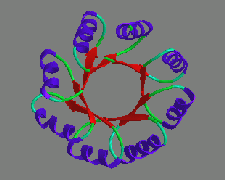
Protein structures are very beautiful, and infinitely complex. There are
people who claim they can calculate the structure of a protein using physical
and mathematical techniques. A simple "back of the enveloppe" calculation
will make clear that this is not possible: The average amino acid has four
free rotable bonds that each have, on average, two and a half local minima
in their conformational space. A protein like BaBa has about 250 amino acids.
The number of possible conformations is thus more than astronomically big.
Finding the correct structure from among so many possible ones makes me dream
about finding that needle in the haystack as a nice and simple task.
Nevertheless, we once tried, and ab initio designed BaBa. We didn't
even do that bad. We got the percentage helix right, and several of the
residues predicted in helices, actually seemed to sit in helices. But the
real structure never became as beautiful as my model!
This illustrates that there is scope for very much research into the
fundamental rules that govern protein folding, structure, and stability.
|
Baba has about 100 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000 000
000 000 000 000 000 possible conformations..... (don't
pin me on a factor of two or so).
|
Fortunately, we do not need to ab initio calculate structures because nature,
crystallographers, and NMR spectroscopists provide us with examples from
which we can extract information that can get us close to reality. we know
this is cheating, but it works! Borrowing ideas from nature is called homology
modelling, and one project that we always work on is improving homology
modelling technology.




